This is a Notice of Liability setting out your personal responsibilities and liabilities for allowing Covid-19 vaccination experimental medical trials on school grounds and or encouraging boys and girls aged 12 years and over to have experimental medications.
It is not my intention to harass, intimidate, offend, conspire, blackmail, coerce or cause anxiety, alarm or distress. This Notice of Liability is presented with honorable and peaceful intentions and is expressly for your benefit to provide you with due process and a good faith opportunity to remedy this most serious matter.
This legal and lawful notice of liability may be used as evidence in court if needed and intends to enlighten you and protect you from attracting civil and criminal liability whether domestic or international and whether in an existing court or one to be convened under Natural Law principles in relation to your action(s) and all your omissions in relation to the alleged SARS-CoV-2 pandemic and the measures that have been, and or are being, taken within the United Kingdom and world-wide to control its alleged spread and effect(s) including, but not limited to, allowing the administration of experimental COVID-19/SARS-CoV-2 mRNA modRNA gene therapies injections vaccines (and or viral vector injections vaccines) on school premises and/or encouraging boys and girls aged 12 years of age and over to have the said experimental gene therapies and the harm and death caused.
You may be held personally liable for and or privately liable for and or civilly and or criminally liable for participating in unlawful, illegal and or criminal activity and or for supporting crimes against humanity, genocide, bio-warfare and or failing to prevent acts so defined, including but not limited to acts that are purposely committed as part of a widespread and or
systematic policy, directed against living men and women, and in particular in your case, boys and girls over 12 years of age, committed in furtherance of government policy.
The four Chief Medical Officers of the United Kingdom on the 13thSeptember 2021, advised Her Majesty’s Government to provide COVID-19 vaccination to the 12-15 year age group, against the advice of the Joint Committee on Vaccination and Immunisation (JCVI) upon whose advice has been followed throughout the COVID-19 pandemic.
In a Channel 4 News interview Professor Anthony Harnden (Deputy Chair JCVI, Lay Council Member of the General Medical Council [GMC] and with research interests in infections and paediatrics in primary care) in defending the decision by the JCVI, said that “My responsibility is to children not the Government”2 – a responsibility that you also hold.
The Covid-19 vaccinations are all currently in phase 3 of clinical trials which are due to end at various points throughout 2023 dependent on the vaccine concerned, understandable given that some of the vaccines are using for the first time in humans mRNA (messenger RNA) and modRNA (nucleoside-modified messenger RNA) technology. Notwithstanding the emergency use authorisation for the administration of these experimental medications, the Government is only underwriting the manufacturers of these experimental medications against any liability arising from their administration; I do not believe that the same applies to you, acting on their behalf in advising and or encouraging and or facilitating the administration of these experimental medications to boys and girls aged 12 years of age and over.
The efficacy of the vaccines have been exaggerated by the pharmaceutical companies, as reported in the medical journal, The Lancet *3
“Vaccine efficacy is generally reported as a relative risk reduction (RRR). It uses the relative risk (RR)—ie, the ratio of attack rates with and without a vaccine—which is expressed as 1–RR. Ranking by reported efficacy gives relative risk reductions of 95% for the Pfizer–BioNTech, 94% for the Moderna–NIH, 90% for the Gamaleya, 67% for the J&J, and 67% for the AstraZeneca–Oxford vaccines.
However, RRR should be seen against the background risk of being infected and becoming ill with COVID-19, which varies between populations and over time. Although the RRR considers only participants who could benefit from the vaccine, the absolute risk reduction (ARR), which is the difference between attack rates with and without a vaccine, considers the whole population.
ARRs tend to be ignored because they give a much less impressive effect size than RRRs: 1·28% for the AstraZeneca–Oxford, 1·24% for the Moderna–NIH, 1·19% for the J&J, 0·93% for the Gamaleya, and 0·84% for the Pfizer–BioNTech vaccines.”
The temporary Experimental Use Authorisation (EUA) by the MHRA (UK) has been granted for Pfizer-BioNTech BNT162b2 4 (mRNA drug) even though they have yet to provide 6-month safety follow-up data in subjects aged 12-15 years from study C4591001 5
The words “Voluntary” and “informed” when considering legal and lawful consent of boys and girls aged 12 years and older and when considering if any purported consent from their parents is lawfully and legally valid, could not be more important for you to pay careful attention to.
Attached is an informed consent form which sets out the law relating to informed consent (based on the supreme Court ruling in Montgomery v Lanarkshire Health Board [2015] UKSC 11)7 and which should be gone through with every person (whether adult or child) in order to enable them to provide informed consent.
What full and objective information does the boy or girl or their parent have in order to enable them to give true voluntary and informed consent? For example, have they been made aware of possible alternative treatments for COVID-19 symptoms should they experience them? Have they been made aware of the true likely benefits to the boy or girl and the actual risks of the trial medication on offer? Are they aware that there is currently no objective data available to suggest that the benefits outweigh the risks on a personal basis to any child? Have they been told that the JCVI expressly states that there is too small a marginal benefit from receiving the trial medications, or that parents should wait for six months before making a decision pending availability of data which might inform such benefit?
If the long-term risks and harms are not fully known (which nobody can possibly argue they can, given the experimental nature of these medications), then how can any man, woman, boy or girl weigh up the “benefits” to the individual boy or girl concerned when they are at such a very low risk of harm from COVID-19 itself?
Does the boy or girl know that there are potential impacts on future fertility and that the mRNA and RNA technologies are completely novel technology and experimental on humans with the possibility of unanticipated and unpredictable long term and late onset health effects?
If a boy or girl is influenced or encouraged by their teacher, headteacher, classmates or celebrity adverts as to the trend to have the vaccine in order to “protect others” or because it has been presented that it will be necessary for college, travelling or even attending pop concerts, how can any boy or girl be said to be giving true voluntary consent?
How can you be satisfied that each boy or girl in your care is not allergic to any of the ingredients in the experimental medication?
Do you have a full list of the vaccine ingredients and have you then carried out an individual risk assessment for each boy and girl in your care to ensure the vaccine ingredients are safe for that particular boy or girl which is being either encouraged and or permitted to take the vaccine whilst under your duty of care? If not, how can you be sure that the boy or girl or parent of that boy or girl has given “informed” consent?
Take note “Safe” is defined by Black’s Law Dictionary as
“the amount of exposure that will cause no harm or no damage after exposure”.
Pursuant to the Duty-Holder’s obligations under Section 3 of The Health and Safety at Work Act 19748 and the Health and Safety at Work Regulations 1999, there is a duty to ensure that any person working onsite performs individual risk assessments. In context of Education, a school holds a primary duty of care towards a child (in loco parentis) safeguarding them from potential risks whether on or off school premises (e.g. school activities) – a duty that cannot be delegated to a third-party [R v Associated Octel Co Ltd (1996) 4 All E R 846] *9
It is unlawful, unethical and immoral to facilitate the administration of and or encourage boys and girls, to whom you have a duty of care to safeguard, to be subject to a trial procedure to which they are at risk of injury or death (see below for the reported government figures form the Yellow card scheme).
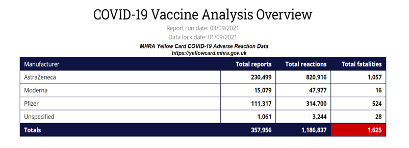
Of relevance to the issue of informed consent is the Yellow Card Scheme10 which the UK Government has established. This System shows that death has been listed 1,625 times as an outcome directly related to COVID-19 vaccines as at September 3, 2021. Deafness as a direct outcome related to COVID-19 vaccines has increased from a minimum of 280 occasions in May 2021 to 1,138 as at September 3, 2021, and Blindness now reported as a direct outcome 495 occasions. It follows that the rates of increase of death and significant harm (excluding blood clotting/strokes/heart attacks are increasing as the vaccination programme is rolled out. As of September 3, 2021 the System shows a total of 1,186,837 adverse reactions to the experimental vaccines. It is a failing as regards informed consent not to make available this information to any boy or girl, man or woman in relation to providing informed consent. The Government estimates that the number of adverse reactions/deaths reported in the Yellow Card System are understated such that the true figure could be 90 – 99% higher than this (i.e. that the figure recorded in the scheme is somewhere between 1 % and 10% of adverse reactions including death on the yellow card scheme), therefore the Government’s own estimate would put deaths anywhere between 16,000 and 160,000.
Fewer children worldwide have died from COVID-19 itself (recorded as death due to a positive result, with or without symptoms, and where underlying health conditions were present and often the cause of death) than those who have died from side-effects from the experimental vaccines. The most common side effects are neurological disorders, blood coagulation/clots and thromboembolic events such as pulmonary embolisms.
Children rarely become symptomatic and are very poor transmitters of COVID-19. Studies prove this and this has not changed. Not one. Children are at no measurable risk from COVID-19 and no previously healthy child has died in the UK after infection.
There is no benefit to a child from the experimental vaccine since they are at no risk from COVID -19, but they are unquestionably at risk from potential side effects and unknown long-term issues.
|
COVID-19 VACCINE DAMAGE – SEPTEMBER 2021 |
|||
|
|
Deaths |
Injuries |
Date |
|
UK |
1,625 |
1,186,837 |
3rd September |
|
EU |
24,526 |
2,317,495 |
11th September |
|
USA |
15,815 |
720,843 |
10th September |
|
TOTAL |
41,966 |
4,225,175 |
|
In addition, on the VAERS *11 USA (Vaccine Adverse Events Reporting System) Death has been listed as an outcome related to COVID-19 vaccines at least 3,924 times as of May 8, 2021 a figure which at September 10, 2021 had risen to 15,815.
adverse reactions. times as of September 11, 2021 and includes 2,317,49524,526 On the European database EndraViligance Death has been listed as an outcome related to COVID-19 vaccines at least

“Myocarditis and Pericarditis …
The observed risk is highest in males 12 through 17 years of age” 13
Absent emergency authorisation which is being used by the UK Government and others around the world to roll out the experimental vaccines, these medications would have to be withdrawn from the “market”. In the USA, for example, deaths in relation to other vaccines numbering as few as 50 (in a country with a population in excess of 360 million) would cause withdrawal of the relevant medication. Comparable provisions apply in the UK and in Europe. This too is something directly relevant to informed consent, as is the data which shows that children who participated in the Pfizer covid vaccine clinical trials have had an adverse reaction rate at 86%12
There is a wider acknowledged link to heart conditions (myocarditis/ myopericarditis and pericarditis) in young men and boys globally, in the US the VAERS reporting system (as of 20 August) has captured 5,093 reports of myocarditis for under 20 year olds11
The US Food and Drug Administration (FDA) documentation acknowledges that in the case of the Pfizer-BioNTech “There is no information on the co-administration of the Pfizer-BioNTech COVID-19 Vaccine with other vaccines”.14 Therefore the risks and potential harm arising from co-administration with say HPV or Influenza “Flu” vaccines for example are unquantified at this time.
It will be noted then that the stance of the NHS as regards the issue of consent is inadequate when compared with provision of fully informed consent, as shown in the attached document which sets out the law relating to informed consent which should be gone through to ensure informed consent is being given. NHS Guidance limits the advice to be provided in relation to “informed consent” to communication of “the anticipated benefits of vaccination in the simplest of terms”, “the likely side effects from vaccination and any individual risks they may run should be addressed”, and “the disbenefits of not consenting to the vaccination”.
Principle 5 of the Nuremberg Code6 states that no medical experiments or trials should be conducted where there is an a priori (theoretical) reason to believe that death or disabling injury will occur. You will appreciate that these medical experiments (the trials for which conclude in 2023) are not theoretical as regards death or disabling injury: there is clear evidence of both arising.
As you are undoubtedly aware, children under the age of 16 can sometimes be deemed “Gillick competent” (for example, a 15 year old girl seeking the contraception pill without her parents’ knowledge) but in relation to experimental medications such as these COVID-19 vaccines which have no long-term data known and where deaths and serious adverse reactions are recorded but not known to the child prior to their “consent” it would be absurd to claim that they were in fact “Gillick competent”. The Court of Appeal case of Bell v Tavistock [2021]17 makes clear that children under 16 years of age need to be deemed Gillick competent by the treating clinician if receiving an experimental medication where the long-term effects may not be clear to the child, the case specifically concerning puberty blockers. Clearly this case means that each child would need to be assessed by the treating clinician as to their informed consent i.e. their understanding of the harm and long-term effects before being given such treatment and of alternative treatments available etc. The case does not amend the Supreme Court judgement of Montgomery v Lanarkshire (2015)7 which sets out what informed consent is.
The case of AB v CD & Others [2021]*16 makes it clear that the absence of Gillick competency cannot then be used to allow parents to consent to a child having an experimental medication when the child him/herself does not want it. The law therefore is protective of children under 16 years of age when it comes to experimental medications, as responsible parents should expect, and recognises children’s limitations regarding being “informed”.
You have a duty of care, and a duty to do no harm and to prevent harm.
Teachers hold a high degree of trust with pupils in that what a teacher says or presents is assumed to be correct by a pupil, an imparting of knowledge that is unbiased, factual, and ethical.
It is well established and accepted in Erikson’s stages of psychosocial development15 that boys and girls in the 12 to 16 year age group are a key developmental stage, where identity and a sense of belonging [inclusion] to a peer group is essential. Any representation to boys or girls regards vaccination or immunisation absent of the analysis of the risks to enable informed decision making, especially considering that the Government has announced that they intend to undermine the lawful rights and obligations of Parents to exercise their Parental Responsibility, as enshrined in s3 Children Act 198918, by use of Gillick Competency test.
It is reasonable to assess therefore that a breach of this implied trust and professional ethics occurs when Teachers do not provide boys and girls with the acknowledged risks and potential consequences of an experimental drug, nor the relationship of their individual risks from either catching or spreading SARS-CoV-2.
Receipt of this notice shows that you have been made aware that death or other serious injuries are possible outcomes for boys and girls age 12 years and over taking the COVID-19 experimental vaccinations and that you are accepting responsibility for any injuries and or deaths that result from the said experimental vaccinations should you or those reporting to you in an employment and or contracting capacity encourage or indeed facilitate boys and girls age 12 years and over to receive experimental COVID-19/SARS-CoV-2 mRNA/modRNA gene therapies/injections/vaccines (and or viral vector injections/vaccines).
In conclusion, given the clear evidence that serious harm (or worse) can and does arise as a consequence of these experimental vaccines given to men, women, boys and girls, those involved at schools (whether head teacher, governors or staff) involved in the process of encouraging or allowing the administration of COVID-19 vaccinations to boys and girls aged 12 years and over, whether directly or indirectly, render themselves liable to criminal prosecution for assault/wounding (or worse if death results) before the domestic courts, in addition to liability for prosecution before the International Criminal Court for breaches of the Nuremberg Code. This is quite separate to any civil liability that arises, or any prosecution for offences contrary to common law.
I therefore seek your reassurance that you will ensure that any children will not be vaccinated on school premises and neither will there be any encouragement for them to receive any COVID-19 or other experimental medications from staff, assuming that the school is content to be involved in any way at all in what amount to clinical trials.
I would also encourage you to send a letter to all parents/guardians informing them of the points raised above, so as to suitably inform any decisions they may take concerning their own children’s health.
1. https://www.gov.uk/government/news/jcvi-issues-updated-advice-on-covid-19-vaccination-of-children-aged-12-to-15
2. Channel 4 Interview with Prof. Harnden [video] https://1drv.ms/v/s!AiMaGiZiw61Xnm44LVRTde5o1OkP
3. COVID-19 vaccine efficacy and effectiveness—the elephant (not) in the room - https://www.thelancet.com/journals/lanmic/article/PIIS2666-5247(21)00069-0/fulltext
4. Pfizer-BioNTech COVID-19 mRNA Vaccine BNT162b2 Phase 3 trial end date: 2 May 2023 https://clinicaltrials.gov/ct2/show/NCT04368728
6. The ten points of the Nuremberg Code
The ten points of the code were given in the section of the judges' verdict entitled "Permissible Medical Experiments"
1. The voluntary consent of the human subject is absolutely essential.
2. The experiment should be such as to yield fruitful results for the good of society, unprocurable by other methods or means of study, and not random and unnecessary in nature.
3. The experiment should be so designed and based on the results of animal experimentation and a knowledge of the natural history of the disease or other problem under study that the anticipated results will justify the performance of the experiment.
4. The experiment should be so conducted as to avoid all unnecessary physical and mental suffering and injury.
5. No experiment should be conducted where there is an a priori reason to believe that death or disabling injury will occur; except, perhaps, in those experiments where the experimental physicians also serve as subjects.
6. The degree of risk to be taken should never exceed that determined by the humanitarian importance of the problem to be solved by the experiment.
7. Proper preparations should be made and adequate facilities provided to protect the experimental subject against even remote possibilities of injury, disability, or death.
8. The experiment should be conducted only by scientifically qualified persons. The highest degree of skill and care should be required through all stages of the experiment of those who conduct or engage in the experiment.
9. During the course of the experiment the human subject should be at liberty to bring the experiment to an end if he has reached the physical or mental state where continuation of the experiment seems to him to be impossible.
10. During the course of the experiment the scientist in charge must be prepared to terminate the experiment at any stage, if he has probable cause to believe, in the exercise of the good faith, superior skill and careful judgment required of him that a continuation of the experiment is likely to result in injury, disability, or death to the experimental subject.
Source: Permissible Medical Experiments. Trials of War Criminals before the Nuremberg Military Tribunals under Control Council Law No. 10: Nuremberg October 1946–April 1949. Washington: U.S. Government Printing Office (n.d.), vol. 2, pp. 181-182.
https://catalog.gpo.gov/F/?func=direct&doc_number=000786119&local_base=GPO01PUB;
https://www.loc.gov/rr/frd/Military_Law/pdf/NT_war-criminals_Vol-II.pdf
7. Legal basis for Informed Consent: https://www.supremecourt.uk/cases/docs/uksc-2013-0136-judgment.pdf
8. HASAWA Section 3: https://www.legislation.gov.uk/ukpga/1974/37/section/3
9. HASAWA relevant case law https://publications.parliament.uk/pa/ld199697/ldjudgmt/jd961114/octel01.htm -
10. YELLOW CARD SYSTEM REPORTS (UK)
a. Website of vaccine reported adverse events - https://coronavirus-yellowcard.mhra.gov.uk
b. Sample of Pfizer reported adverse events - https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/986035/DAP_Pfizer_050521.pdf
c. Sample of Astra Zeneca reported adverse events - https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/986033/DAP_AstraZeneca_050521.pdf
d. Sample of Moderna reported adverse events - https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/986034/DAP_Moderna_050521.pdf
e. Sample of unspecified reported adverse events - https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/986036/DAP_Unspecified_050521.pdf
Run your own report to check results here by clicking link below and follow instructions:
https://wonder.cdc.gov/vaers.html
Instructions for use
Click ‘I agree’
Click ‘Data Report’
Choose from section 1. ‘Group results by - Vaccine manufacturer’
Choose from section 3. ‘Vaccine products - Covid 19 vaccines’
Choose from section 4. ‘Location – All Locations’
Choose from section 5. ‘Event category - Death’
Scroll to bottom of page and press ‘Send’
View latest data for deaths reported from Covid Vaccines grouped by Vaccine manufacturer
12. https://dailyexpose.co.uk/2021/05/30/shocking-86-of-children-suffered-an-adverse-reaction-to-the-pfizer-covid-vaccine-in-clinical-trial/; (https://www.afinalwarning.com/522797.html); https://www.fda.gov/media/144413/download
13. Myopericarditis following COVID-19 vaccination: Updates from the Vaccine Adverse Event Reporting System (VAERS) [Aug 30, 2021] https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-08-30/03-COVID-Su-508.pdf
14. FDA Pfizer-BioNTech information: https://www.fda.gov/media/144413/download
15. Erikson’s Stages of Psychosocial Development https://www.ncbi.nlm.nih.gov/books/NBK556096/
16. AB v CD & Others [2021] https://www.bailii.org/ew/cases/EWHC/Fam/2021/741.html
17. Court of Appeal case of Bell v Tavistock [2021] https://www.judiciary.uk/wp-content/uploads/2021/09/Bell-v-Tavistock-judgment-170921.pdf
18. Children Act 1989 – Section 3 https://www.legislation.gov.uk/ukpga/1989/41/section/3
Dr Alasdair P S Munro, NIHR Southampton Clinical Research Facility and NIHR Southampton Biomedical Research Centre, University Hospital Southampton NHS Foundation Trust: https://adc.bmj.com/content/105/7/618
Contingency planning in place to vaccinate secondary school pupils at start of new academic year”
https://www.bmj.com/content/371/bmj.m4058
https://www.imperial.ac.uk/covid-19-vaccine-trial/
Purpose:
This form has been designed to support the Informed Consent process for Covid-19 vaccinations.
FOR THE LEGAL ADMINISTRATION OF ANY CV19 VACCINE, BOTH PARTIES MUST READ AND SIGN THIS DOCUMENT
Audience:
• Doctors (or their delegated Health Care Professionals)
• Patients receiving Covid-19 Vaccine
Background:
This document is based on the Montgomery Judgement and GMC Guidelines.
The Montgomery Judgement and Informed Consent
https://www.themdu.com/guidance-and-advice/guides/montgomery-and-informed-consent
This Supreme Court judgement of Montgomery v Lanarkshire (2015) changed the standards of consent. The key passages from Montgomery Judgement state:
“...The doctor is therefore under a duty to take reasonable care to ensure that the patient is aware of any material risks involved in any recommended treatment, and of any reasonable alternative or variant treatments....”
“The test of materiality is whether, in the circumstances of the particular case, a reasonable person in the patient's position would be likely to attach significance to the risk, or the doctor is or should reasonably be aware that the particular patient would be likely to attach significance to it.”
. This puts the patient at the centre of consent process, as their understanding of material risk must be considered. Both patient and doctor need to sign this document. If doctors fail to properly discuss the risks and alternative treatments with the patient, this renders them personally responsible for damages. This document therefore protects the patient and the doctor.doctors must provide information about all material risks to which a reasonable person in the patient's position would attach significanceBefore Montgomery, a doctor's duty to warn patients of risks was based on whether they had acted in line with a responsible body of medical opinion - known as the “Bolam test”. Now,
General Medical Council Guidance - Decision Making and Consent (2020)
(https://www.gmc-uk.org/ethical-guidance/ethical-guidance-for-doctors/decision-making-and-consent )
This states that doctors MUST attempt to find out what matters to patients, so they can share information about the benefits and harms of proposed options and reasonable alternatives. Note the word MUST makes this a legally binding directive. GMC Guidance states doctors MUST address the following information:
a) Recognise risks of harm that you believe anyone in the patient’s position would want to know. You’ll know these already from your professional knowledge and experience.
b) The effect of the patient’s individual clinical circumstances on the probability of a benefit or harm occurring. If you know the patient’s medical history, you’ll know some of what you need to share already, but the dialogue could reveal more.
c) Risks of harm and potential benefits that the patient would consider significant for any reason. These will be revealed during your discussion with the patient about what matters to them.
d) Any risk of serious harm, however unlikely it is to occur.
e) Expected harms, including common side effects and what to do if they occur.
“The discovery and research phase is normally two-to-five years, according to the Wellcome Trust. In total, a vaccine can take more than 10 years to fully develop”
https://www.weforum.org/agenda/2020/06/vaccine-development-barriers-coronavirus/
Vaccines trigger post viral syndromes:
“We present epidemiological, clinical and experimental evidence that ME/CFS constitutes a major type of adverse effect of vaccines” (2019 paper)
https://www.sciencedirect.com/science/article/abs/pii/S1568997219301090
Allergy and autoimmunity effects of vaccines:
|
1. Shoenfeld Y et al - Vaccination and autoimmunity - Vaccinosis: A dangerous liaison? J Autoimun 2000;14:1-10. 2. Nossal GJV - Vaccination and autoimmunity. JAI 2000;14:15-22. 3. Shoenfeld Y et al - Vaccination as an additional player in the mosaic of autoimmunity. Clin Exp Rheumatol 2000;18 4. Rogerson SJ. Nye FJ - Hepatitis B vaccine associated with erythema nodosum and polyarthritis. BMJ 1990;301:345. 5. Haschulla E et al - Reactive arthritis after hepatitis B vaccination. J Rheumatol 1990;17:1250-1251. 6. Biasi D et al - A new case of reactive arthritis after hepatitis B vaccination. Clin Exp Rheumatol 1993;11:215. 7. Gross K et al - Arthritis after hepatitis B vaccination. Report of three cases. Scand J Rheumatol 1995;24:50-52. 8. Maillefert JF et al - Rheumatic disorders developed after hepatitis B vaccination. Rheumatology (Oxford) 1999;38:978-983 |
9. Grasland A et al - Adult-onset Still's disease after hepatitis A and B vaccination (article in French). Rev Med Interne 1998;19:134-136. 10. Pope JE et al - The development of rheumatoid arthritis after recombinant hepatitis B vaccination. J Rheumatol 1998;25:1687-1693. 11. Guiseriz J - Systemic lupus erythematosus following hepatitis B vaccine. Nephron 1996;74:441. 12. Grezard P et al - Lupus erythematosus and buccal aphthosis after hepatitis B vaccination in a 6-yearold child. Ann Dermatol Vener 1996;123:657-659. 13. Weibel RE et al - Chronic arthropathy and musculoskeletal symptoms associated with rubella vaccines. A review of 124 claims submitted to the National Vaccine Injury Compensation Program. Arthritis Rheum 1996;39:1529-1534. 14. Ray P et al - Risk of chronic arthropathy among women after rubella vaccination. Vaccine Safety Datalink Team. JAMA 1997;278:551-556. 15. Howson CP et al - Adverse events following pertussis and rubella vaccines. Summary of a report of the Institute of Medicine. JAMA 1992;267;392-396. |
16. Howson CP et al - Chronic arthritis after rubella vaccination. Clin Infect Dis 1992;15:307-312. 17. Mitchell LA et al - HLA-DR class II associations with rubella vaccine-induced joint manifestations. J Infect Dis 1998;177:5-12. 18. Nussinovitch M, Harel L, Varsano I. Arthritis after mumps and measles vaccination. Arch Dis Child 1995;72:348-349. 19. Thurairajan G et al Polyarthropathy, orbital myositis and posterior scleritis: an unusual adverse reaction to influenza vaccine. Br J Rheumatol 1997;36:120- 123. 20. Maillefert JF et al - Arthritis following combined vaccine against diphtheria, polyomyelitis and tetanus toxoid. Clin Exp Rheumatol 2000;18:255-256. 21. Adachi JA et al - Reactive arthritis associated with typhoid vaccination in travelers: report of two cases with negative HLA-B27. J Travel Med 2000;7:35-36. 22. Older SA et al - Can immunization precipitate connective tissue disease? Report of five cases of systemic lupus erythematosus and review of the literature. Sem Arthritis Rheum 1999;29:131-139 |
References
| Montgomery Judgement & GMC Guidance |
Facts |
Notes |
Discussed |
|
|
2015 Montgomery Judgement on Informed Consent |
The doctor is therefore under a duty to take reasonable care to ensure that the patient is aware of .......... any reasonable alternative or variant treatments. |
Vitamin D, 5,000iu daily has proven benefit to prevent and treat Covid-19 Vitamin C, 5 grams daily has proven benefit to prevent and treat Covid-19 Topical antiseptics (such as iodine) are of proven benefit to reduce the loading dose, and hence disease severity, of Covid-19 Ivermectin and Hydroxychloroquine are available alternative medications for prophylaxis and or treatment of COVID-19. Individual medical practitioners who are licensed to prescribe Ivermectin, for example, have been advised by the MHRA in writing that they are permitted to do so if their clinical judgment is such that this is an appropriate course to take have undertaken the appropriate clinical assessment of a patient. |
Yes/no |
|
|
GMC Guidelines to Doctors |
Facts |
Notes |
Discussed |
|
|
a. Recognised risks of harm that you believe anyone in the patient’s position would want to know. You’ll know these already from your professional knowledge and experience. |
CV-19 vaccine development accelerated. Vaccine safety testing normally c.10 years. Current CV-19 vaccines trialled for a few months with little/no animal
testing. PHASE 3 trials won’t complete for 2 years https://www.bmj.com/content/370/bmj.m3096/rr https://www.bulatlat.com/2020/08/21/hazards-of-the-covid-19-vaccine/ CV-19 vaccines may sensitise recipients to more severe disease https://doi.org/10.1111/ijcp.13795
|
Yes/no
|
||
|
|
There have been reports of some serious side effects including 2 cases of transverse myelitis and neurological conditions in the Astra Zeneca vaccine trial. |
Astra Zeneca Transverse Myelitis report https://www.nature.com/articles/d41586-020-02594-w https://www.nytimes.com/2020/09/19/health/astrazeneca-vaccine-safety-blueprints.html |
Yes/no
|
|
|
Facts |
Notes |
Discussed
|
||
|
continued |
Anaphylaxis reports: Preparations to manage anaphylaxis vaccine recipients:
|
Yes/no
|
||
|
b. The effect of the patient’s individual clinical circumstances on the probability of a benefit or harm occurring. |
It is known that vaccines can switch on allergy and autoimmunity. May be contraindicated with pre-existing autoimmune conditions or CFS/ME, or previous vaccine injury/reactions. MHRA 09 December 2020: Any person with a history of anaphylaxis to a vaccine, medicine or food should not receive the Pfizer/BioNTech vaccine. A second dose should not be given to anyone who has experienced anaphylaxis following administration of the first dose |
Any patient with a history or strong family history of allergies or autoimmune conditions may choose to refuse a CV-19 vaccine. Doctors working with CFS/ME patients already advise them to avoid vaccination as this may trigger a relapse. |
Yes/no
|
|
|
c. Risks of harm and potential benefits that the patient would consider significant for any reason. These will be revealed during your discussion with the patient about what matters to them. |
Patient’s individual risk from Covid-19 MUST be discussed – IFR <0.05% for <70 years to weigh up against risk from vaccine. Patient expectation of vaccine benefit i.e. reducing risk of severe illness, hospitalisation and preventing infection with and transmission of SARS-Cov-2 Patients MUST be made aware of the full list of vaccine ingredients |
Covid-19 IFR estimate by age (Table 2): https://spiral.imperial.ac.uk:8443/bitstream/10044/1/83545/8/2020-10-29-COVID19-Report-34.pdf Make patient aware that current trials are not designed to show if CV-19 vaccine will reduce their risk of hospitalisation or death or will prevent infection and transmission of virus as may affect risk v benefit profile https://www.bmj.com/content/371/bmj.m4037 Ethical/religious considerations e.g. animal products - vegetarianism/veganism, WI-38 human diploid cells (aborted fetus source) - pro-life/religious belief |
Yes/no
|
Date and Time
|
|
Name of doctor or Nurse administrating
|
|
Professional number of doctor (GMC) or nurse (GNC)
|
|
Name of vaccine, batch number and date of administration
|
|
Signature
|
|
Date and Time
|
|
Name of Patient
|
|
Name of parent or guardian if consenting on behalf of a child
|
|
Contact phone number or email
|
|
Signature
|
|
|
|
|
|
|
|
Your name *